Search
Search
Our Faculty
-
Faculty Structure Open or Close
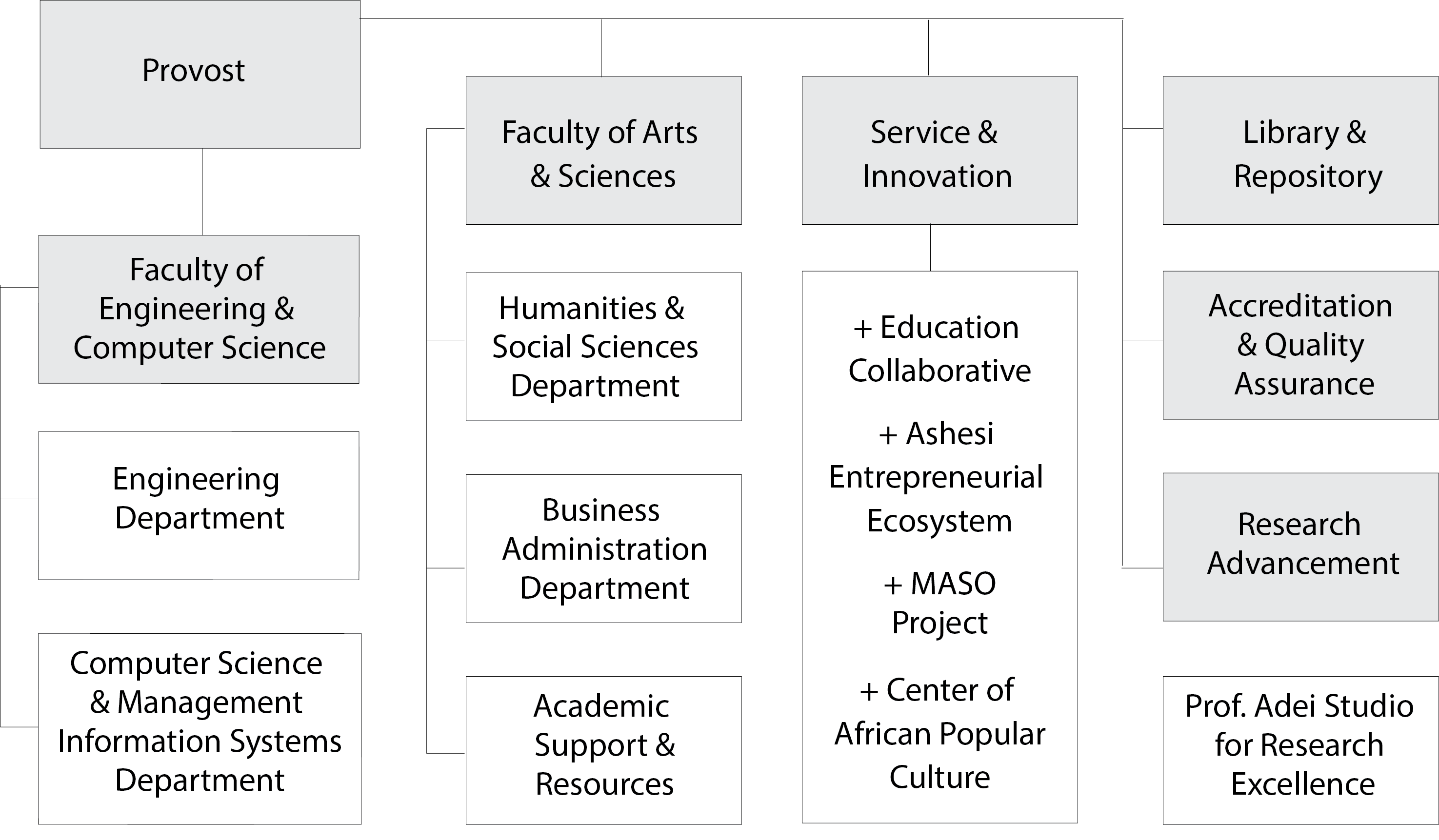
1. Faculty of Business, Arts & Social Sciences:
a. Humanities and Social Sciences Department
This department is responsible for the liberal arts core curriculum.
b. Business Administration Department:
This department is responsible for the Business Administration major and also offers courses towards the Management Information Systems major.
2. Faculty of Engineering and Computer Science:
a. Department of Engineering
The Department of Engineering oversees Mechanical Engineering, Computer Engineering and Electrical & Electronic Engineering.
b. Department of Computer Science and Information Systems (CSIS). CSIS has primary responsibility for overseeing and advising both Computer Science and Management Information Systems students. The Mathematics and Statistics faculty have been brought into the CSIS department. The math and statistics faculty will continue to run courses for all students, including engineering, computer science, management information systems, and business administration students. -
Our Students Open or Close
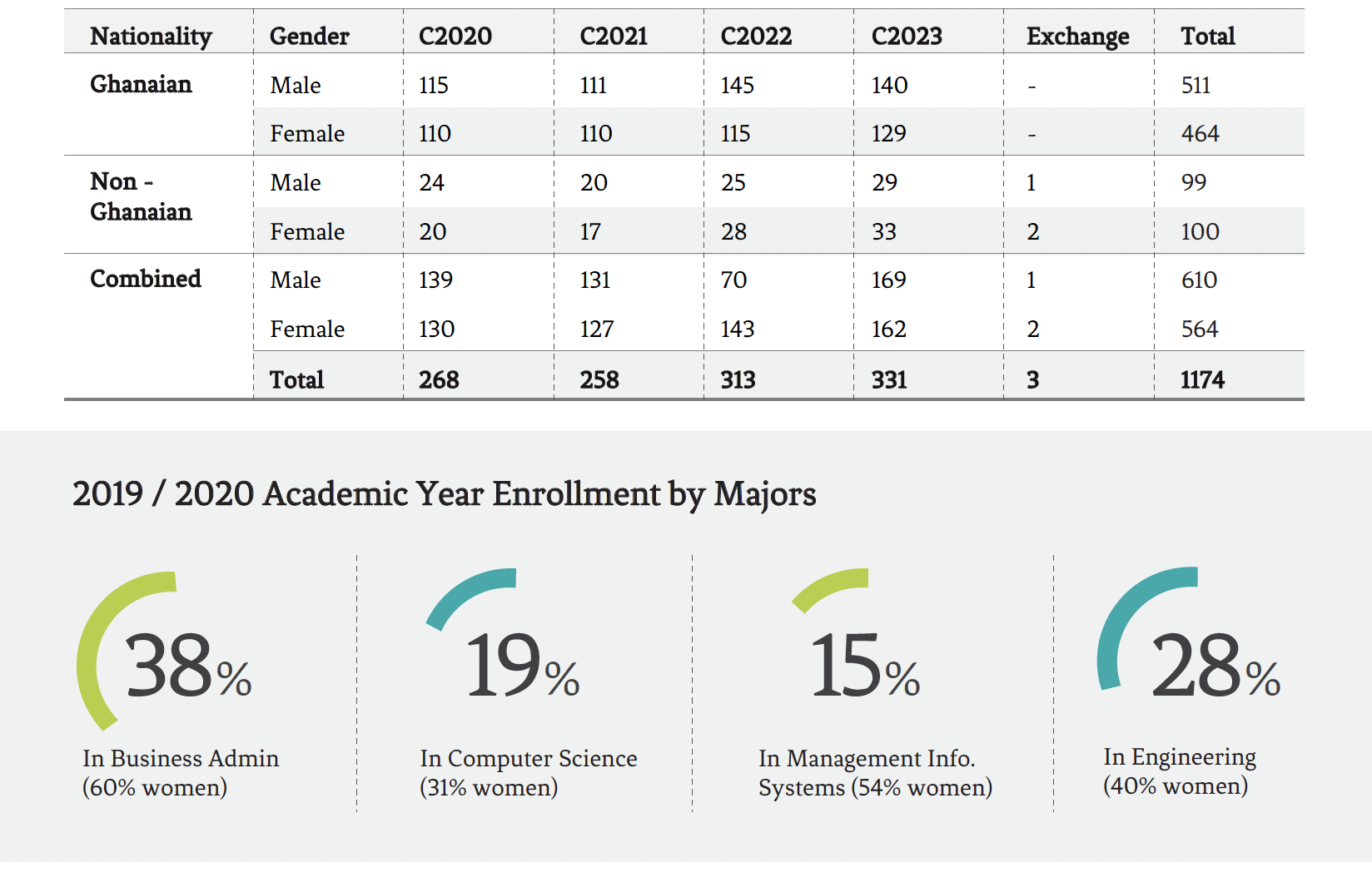
-
Institutional Review Board (IRB) Open or Close
Ashesi Institutional Review Board
The Institutional Review Board (IRB) provides accreditation for research activity across and within the University. The IRB must approve all research conducted by students, faculty, staff, or research affiliates from other institutions before primary data collection or project initiation. The goal of reviews is to ensure that research activity prioritises human subjects' safety and privacy. Undergraduate, Masters & PHD students should download the student application. Ashesi Faculty & Staff, External Bodies, Organisation and Independent Researchers should download the staff application.
-
Application Review Process Open or Close
Human Subjects approval must precede submission of all research applications to any sponsor. This applies to funding requests for internal or external resources, domestic and international applications. This policy applies to all applications including those to for-profit, not-for-profit, governmental or charitable organisations.
Review & Response Time
The committee will respond to each proposal within four weeks following its review. If the Committee determines that a submission involves vulnerable populations or greater than minimal risk, the applicant will have an opportunity to modify the research design, sampling strategies, process or format of data collection, or any other concern that is raised by the Committee. Appeals can be formally addressed by the Provost with the Chair of the Human Subjects Review Committee. If an appeal is approved, the full committee will revisit the proposal and will entertain all concerns of the Principal Investigator. In the event that an appeal is also denied, the proposal must be closed for further consideration unless methodological and ethical issues are adequately addressed in the form of a new submission.
Incomplete Application
Applications that are incomplete will not be forwarded to the Committee for review. Incomplete applications will be returned to the applicant. Applicants are advised to be mindful of schedules and time requirements for the review process and urged to submit proposals as early as possible.
Committee Dispositions
A letter will be sent to applicants as quickly as possible about Committee decisions regarding the status of an IRB Application. Status can be:a. Approval without conditions
b. Conditional approval: pending completion of specific items in the application, clarification of confusing text, or other details as noted by reviewer(s).
c. Disapproval: indicating that the submission was so deficient that the applicant must try again from the beginning.
Faculty or Staff Submissions and External Organisational or Individual Submissions
Faculty (or staff) of Ashesi University, and all externally-sourced applications, must submit completed application forms and appendices to the Chair of the IRB in soft copy form. Faculty and staff applications are accepted throughout the academic year (full semesters). Applications can be submitted during the summer period with prior approval from the Chair. The implication of this approval is that the Chair will be responsible for orchestrating an ‘off season’ review by either members of the Ashesi IRB or competently trained faculty as ad hoc members for a single review.
Archiving and Security
Human Subjects Review documentation is to be secured and preserved for a minimum of seven (7) years after original data collection or subsequent to the first publication based on the collected data, whichever is the longer time period.
Validity Period
All approved / conditionally approved Human Subject Review Applications are valid for two (2) years from the date of validation.
Modifications After Review
Any substantive changes made to the project after approval by the Institutional Review Board should be immediately brought to the attention of the committee by the principal investigator or supervisor and this may necessitate another review. -
Exemptions Open or Close
Investigators do not determine their research to be exempt from review. Only the Review Board may provide exemptions through its review processes. However, some pre-exemptions may be applied to an activity that:
a. is not classified as a research activity. Research is defined as: “A systematic investigation designed to develop or contribute to generalisable knowledge.”
b. does not involve human subjects. A human subject is defined as “a living individual about whom an investigator conducting research obtains (1) data through intervention or interaction with the individual, or (2) identifiable private information.”
Research activities which only involve human subjects in one or more of the following categories may be determined to be exempt from Ashesi University Human Subject Review Committee review. Requests for exemption must cite the statutory basis for the requested exemption from the categories listed below:
I. Research- Research conducted in established or commonly accepted educational settings involving regular education practices.
- Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), if information taken from these sources, is recorded so that subjects cannot be identified, directly or indirectly, through identifiers linked to the subjects.
- Research involving observation of public behaviour or survey or interview procedures for all of the conditions below. This exemption does not apply for research involving children, except where the investigator does not participate in observing activities.
- The research does not place subjects at the risk of civil or criminal liability or damage the subject’s financial standing or employability, AND
- The research does not deal with sensitive aspects of the subject’s behaviour, such as illegal conduct, drug use, sexual behaviour or use of alcohol, AND
- The research does not use materials, procedures or settings likely to be embarrassing, upsetting or intrusive to the subjects, AND
- The subject cannot be identified, and confidentiality is protected.
- All research involving survey or interview procedures is exempt, without exception, when the subject is an elected or appointed official or a public office candidate.
- Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens (if these sources are publicly available or if the information is recorded in such a manner that subjects cannot be identified directly or indirectly through identifiers linked to the subject).
II. Program Review
- Government Regulations Exemptions. Research and demonstration projects that are conducted by or subject to the approval of Ghana government department or agency heads, and that are designed to study, evaluate, or otherwise examine 1) public benefit or service programs; 2) procedures for obtaining benefits or services under those programs; 3) possible changes in or alternatives to those programs or procedures; or 4) possible changes in methods or levels of payment for benefits or services under those programs.
Note: The above exemptions do not apply to research involving venerable groups like prisoners or institutionalised patients as subjects.


